1. It's a story of light, but first from the story of Rubisco
The Calvin-Benson cycle is driven by ATP and NADPH made of chloroplast thylakoid membranes. The protagonist of this circuit, ribulose-1,5-bisphosphate carboxylase / oxygenase (commonly known as Rubisco), is a 16-molecular protein consisting of 8 large subunits of the chloroplast code and 8 small subunits of the nuclear code. The total molecular weight of the molecule amounts to 550,000. The active site is located in each major subunit, and ribulose-1,5-bisphosphate (RuBP), which is a pentacarbonic acid diphosphate, is first incorporated into the active site, and CO2 and O2 attack it competitively. The CO2 fixation rate when CO2 is saturated is about 3 mol CO2 mol-1 active site s -1. This rate is 2-3 orders of magnitude lower than that of general enzymes. Rubisco's Km for CO2 is 10 to 20 µM at 25 ° C. The CO2 concentration of 12.3 µM in water at 25 ° C, which is in equilibrium with the current atmospheric CO2 concentration (390 ppm), is approximately equal to Km. In the presence of 21% O2, the apparent Km is about 1.5 times. Since CO2 in the atmosphere diffuses into the leaves according to the concentration gradient, the CO2 concentration in the chloroplast is lower than 390 ppm as described later. Therefore, the actual CO2 fixation rate performed inside the leaves is at most 1 mol mol-1 active site s-1. In the Calvin-Benson circuit, when one molecule of CO2 is fixed, sugar equivalent to one carbon (1/3 triose phosphate) is produced and RuBP is regenerated. 3 ATP and 2 NADPH are used to drive this circuit.
On the other hand, when RuBP is incorporated into the active site of Rubisco and O2 attacks it, phosphoglycolate, which is a C2 compound, is produced in addition to the product phosphoglycerate when CO2 attacks. This phosphoglycolate is a potent inhibitor of triose phosphate isomerase in the Calvin-Benson cycle. Therefore, the plant must metabolize this promptly. Also, since the carbon contained in phosphoglycolate is also fixed using ATP and NADPH, as much C as possible must be recovered from this molecule. The so-called "photorespiration" pathway meets these two requirements. This pathway is carried out by the collaboration of three organelles: chloroplasts, peroxysomes, and mitochondria. Electron micrographs of these organelles in close proximity to each other are impressive, but recently it has become clear that these organelles move together in cells in the presence of light.
The carbon in Phosphoglycolate is not completely recovered, and 0.5 of the two carbons contained in this molecule are released as CO2. In this pathway, O2 is absorbed during the oxidation of RuBP and the oxidation of glycolate to produce glyoxylate. The latter reaction produces H2O2, while the catalase reaction produces 1/2 O2. The net entry and exit of these gases is the absorption of O2 and the release of CO2, hence the name "photorespiration". Although this name is misleading, photorespiration is a reaction that should be considered as part of the photosynthetic reaction, not directly related to the "breathing" that takes place in mitochondria. In the photorespiration pathway, a single RuBP oxygenation consumes 3.5 ATP and energy equivalent to 2 NADPH. In addition, this route alone will result in the loss of 0.5 carbons from RuBP, which has 5 carbons, so that RuBP cannot be regenerated. If this is supplemented with 0.5 carbon equivalents (1/6 tricarbonate phosphate), an additional 1.5 ATP and NADPH will be consumed. In total, 5 ATP and 3 NADPH will be used.
In this way, when the oxygenation reaction occurs, it consumes a large amount of energy and reducing power, which has a great negative effect on the efficiency of photosynthesis. Therefore, C3 plants should have evolved to suppress photorespiration as much as possible. The most effective way to suppress photorespiration would be to keep the CO2 concentration in the chloroplast as high as possible.
2.CO2 concentration in the chloroplast
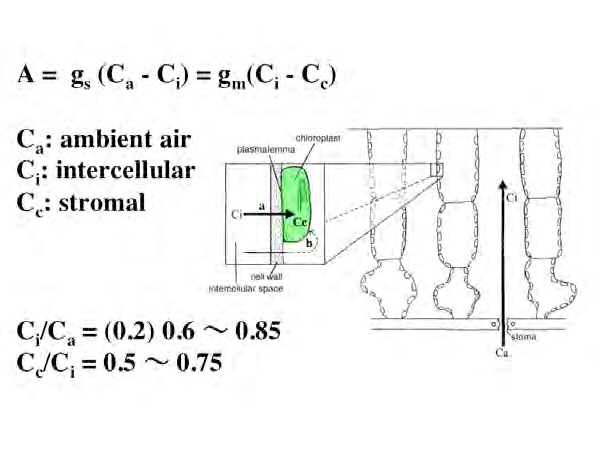
CO2 in the atmosphere diffuses into the leaves through the stomata. This driving force is the difference from the outside air CO2 concentration caused by the decrease in CO2 concentration inside the leaves due to photosynthesis. CO2 diffuses from the stomata into the leaves and diffuses the intercellular gas phase. It then dissolves in the apoplast water of the cell wall and reaches the stroma via the cell membrane, cytosol, and chloroplast envelope. If this CO2 diffusion rate (= photosynthesis rate) is compared to an electric current, the CO2 concentration difference corresponds to the potential difference, and the following equation similar to Ohm's law holds.
Photosynthesis rate = (CO2 concentration difference) / diffusion resistance
The reciprocal of the diffusion resistance is "ease of diffusion of CO2" and is called conductance. The CO2 diffusion conductance can be measured, and the most reliable method of measurement is the method using Rubisco's separation of 13CO2 and 12CO2. If Rubisco is in a completely open system, 12CO2 will be fixed in preference to 13CO2. On the other hand, if it is in a completely closed system, both will be fixed. The inside of the leaf is an intermediate state between these. Due to Rubisco's own isotope fractionation, 13CO2 / 12CO2 around Rubisco is higher than 13CO2 / 12CO2 in the open air. And Rubisco fixes the corresponding 13CO2. When the conductance is small and CO2 is difficult to diffuse, the fixed CO2, 13CO2 / 12CO2, becomes large.
In practice, at the same time as the normal gas exchange measurement, air is sampled before and after the assimilation box and the ratio of 13CO2 to 12CO2 is measured with a mass spectrometer. Recently, Tunable Laser Diode Absorption Spectroscopy has made it possible to directly measure the concentrations of 12CO2 and 13CO2 in the air.
Figure 1 outlines the results of such measurements.
If Ca, Ci, and Cc are air, intercellular, and stroma CO2 concentration, gs is stomatal conductance, and gm is mesophyll conductance, the photosynthetic rate A of leaves can be expressed by the following equation.
Since Ci / Ca takes values on the same order as Cc / Ci, mesophyll conductance, like stomatal conductance, is a very important rate-determining factor in photosynthesis.
The factors that determine mesophyll conductance have also become clear. One is the value (Sc) obtained by dividing the cumulative surface area of the chloroplast facing the intercellular region by the leaf area. Since the rate at which CO2 diffuses in the liquid phase is only about 1 / 10,000 of that in the gas phase, the place where CO2 dissolves inside the leaf is the part where the shortest distance can be taken, that is, the chloroplast passes through the cell membrane / cell wall. It is the part that touches the intercellular region. In fact, most chloroplasts are located in contact with the cell wall. Senn pointed out this in 1905 and also mentioned the possibility of CO2 chemotaxis in chloroplasts. However, little research has been done on the CO2 taxis of chloroplasts.
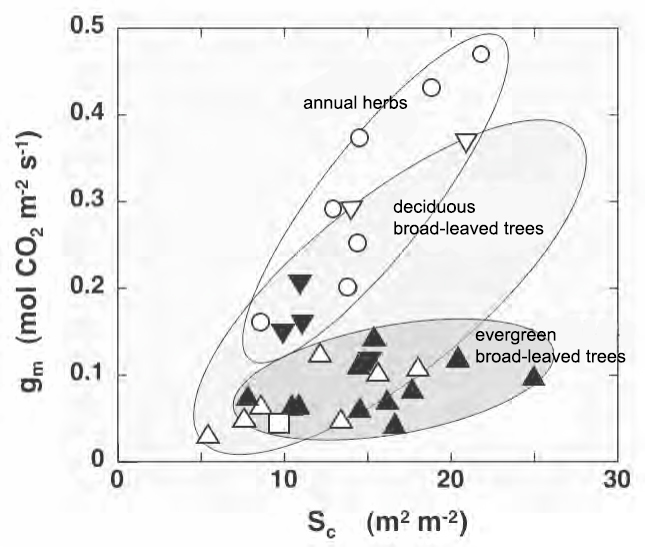
Fig. 2 Dependence on cumulative chloroplast surface area (Sc) of mesophyll conductance modified)
○, annual herbs; △, deciduous broad-leaved trees; ▲ ▼, evergreen broad-leaved trees. Mainly based on data from Yuko Hanba et al.
Figure 2 is a plot of the mesoconductance gm obtained so far against Sc. In this way, the data varies widely. However, when summarized by ecotypes such as annual herbs, deciduous broad-leaved trees, and evergreen broad-leaved trees, the dependence on Sc can be seen. In addition, when the chloroplast is localized to a strong light position by strong blue light and the Sc is changed, the gm changes accordingly, and the Sc increases when the mature leaves are acclimatized to the strong light. The importance of Sc can be seen from the fact that the maximum photosynthetic rate increases accordingly. The difference in inclination depending on the ecotype in Fig. 2 can be explained by the difference in cell wall thickness of mesophyll cells. For example, the cell wall thickness of mesophyll cells of annual herbs, deciduous broad-leaved trees, and evergreen broad-leaved trees is 0.1 to 0.2, 0.2 to 0.3, and 0.3 to 0.4 µm.
It has also become clear that the cell membrane protein aquaporin is involved in controlling gm. The author proposes the name cooporin because it allows CO2 to permeate and probably cooperates with carbonic anhydrase.
3. The meaning of leaf thickness
Leaves in a bright light environment must have a large amount of "big and slow" Rubisco in order for their photosynthetic activity to be high enough. At that time, Rubisco cannot be packed tightly in the cells. In order to suppress the oxygenation activity of Rubisco and fix CO2 at the highest possible rate, it is necessary to reduce the Rubisco concentration per Sc and thin the chloroplast so that it sticks to the cell membrane. That is, Sc must be increased and mesoconductance must be increased. In order to increase Sc, the cell surface area must be increased. If the cell diameter does not change, the leaves must be thickened.
4. Why the leaves are not black: Optical properties of the leaves
In order to absorb and use sunlight efficiently, it seems ideal to make black leaves. However, it is only when the energy of the absorbed light is converted into chemical energy very efficiently. As already mentioned, in order for the leaves to receive strong light and perform sufficient photosynthesis, they must have a considerable amount of chloroplasts containing a large amount of Rubisco. In addition, in order to supply Rubisco with as high a concentration of CO2 as possible, chloroplasts must be arranged along the intercellular form. And, in order to perform efficient photosynthesis, sufficient light must be supplied to all of these chloroplasts. In other words, the leaves must simultaneously meet the seemingly contradictory requirements of absorbing as much light as possible and distributing light as evenly as possible.
It is often said that "the leaves look green because the leaves do not absorb green light" and "the leaves do not absorb green light and are not used for photosynthesis". However, the leaves absorb a lot of green light, and the absorbed green light is efficiently used for photosynthesis.
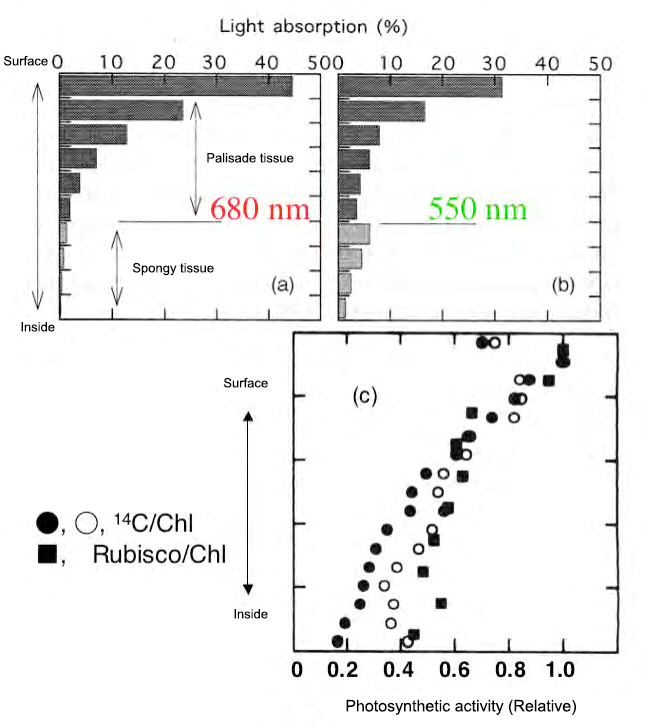
Fig. 3 Light absorption gradient and photosynthetic ability gradient inside the leaf
(a), (b), Absorption rate (Terashima and Saeki, 1985) calculated based on the measurement of transmittance and reflectance of the leaf section of Yabutsubaki (Terashima and Saeki, 1983) was drawn based on the modification. The chlorophyll content of each layer is 0.5 mg dm-2 (approximately 0.056 mmol m-2).
(C) His Rubisco quantification data per chlorophyll in spinach sections (Terashima and Inoue, 1985) and his data on spinach leaves uptake with 14 CO 2 under saturated light (Vogelmann et et al.) al. 1995) was also drawn.
Since a leaf is composed of cells having a refractive index of about 1.5 and air contained in an intercellular cell having a refractive index of 1.0, the light incident on the leaf moves back and forth inside the leaf. The light absorption rate increases as the light encounters the chloroplasts many times. This effect is of little use in increasing the absorption rate of blue and red light, which is mostly absorbed by encountering chloroplasts once, but not so much by encountering chloroplasts once. Significantly increases the absorption rate of green light. As a result, in general green leaves, the absorption rate of blue light and red light is about 90%, while the absorption rate of green light is also about 70 to 80%. In addition, by about 1970, many action spectra of photosynthesis measured under low light conditions had been obtained, and it is known that once absorbed, green light also drives photosynthesis with high efficiency.
5. Light environment inside the leaf and the construction principle of the photosynthesis system
The author has conducted research by Monsi and Saeki (1953) applying the method of analyzing the relationship between the light environment of the leaf group and the photosynthesis of the community to the leaf level.
Figures 3- (a) and (b) show the leaves calculated from the measured transmittances and reflectances of the tissue sections of the yabutsubaki obtained from the typical dorsoventral lobe with palisade and spongy tissues. The light absorption pattern inside is shown. Each layer is set so that the amount of chlorophyll per area is equal. When irradiating light from the front side, most of the light with a strong absorption wavelength such as 680 nm (red light) is absorbed by the upper part of the fence-like tissue. On the other hand, a significant portion of the 550 nm green light reaches the spongy tissue. The spongy tissue also absorbs 550 nm light well because it scatters light and increases the chances of encountering chloroplasts. The presence of spongy tissue on the back side of the leaf can be said to be an extremely advantageous property for absorbing green light inside the leaf.
Since the palisade tissue is in close contact with the epidermis, light is easily transmitted through the leaves and the reflection is small. The color of the front side of the leaves with clear front and back is dark because the reflection is suppressed on the front side. On the other hand, the back side of the leaf looks whitish because the amorphous cells of the spongy tissue are not so close to the epidermis that the light incident from the back side is "prepaid" before encountering the chloroplast. Is. The high reflectance on the back side is a reflection of the morphology of the spongy tissue, which helps to absorb the light incident from the front side under natural conditions, not the property of reflecting the light shining from the back side.
Since the chloroplasts adapt to the light environment with respect to the gradient of the amount of light absorption formed inside the leaf, a gradient of positive chloroplast to negative chloroplast is formed inside the leaf (Fig. 3- (Fig. 3- (Fig. 3- (Fig. 3- (Fig. 3-)). c)). Leaf tissue differentiation and chloroplast acclimation act in the direction in which all chloroplasts inside the leaf function with high efficiency, which greatly contributes to an increase in the utilization efficiency of light resources and nitrogen resources of the entire leaf. (Explained in detail in Fig. 5).
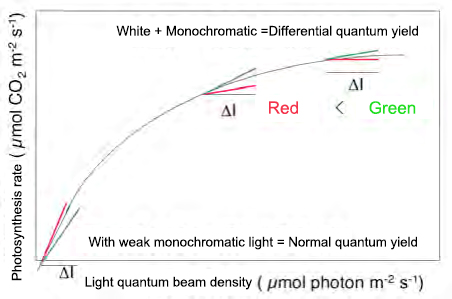
Fig. 4 Modified differential quantum yield measurement method
Whereas the normal photosynthetic spectrum is obtained using weak monochromatic light, this method irradiates weak monochromatic light in the presence of strong white light. When the efficiency is plotted against the irradiation photon flux density, at a very weak photon flux density, red light is more efficient, reflecting the absorption rate of leaves, but when strong white light coexists, green light is more efficient. May be efficient.
However, in the figure of Fig. 3, the gradient of light absorption in the leaves is larger than the gradient of the maximum photosynthetic rate of chloroplasts. Of course, since the species are different, there is no point in comparing Fig. 3 directly, but as far as we have investigated several species of plants so far, the photosynthetic activity is absorbed even in the chloroplasts where the amount of light absorption is small. The general tendency is not to be as low as the decrease in volume. Perhaps the reason is that chloroplasts do not have a very large dynamic range of adaptation to the light environment.
In any case, in many green leaves, when the light becomes stronger, the photosynthesis of the chloroplast on the front side is photosaturated first, and the chloroplast near the back side does not reach the light saturation. When the intensity of white light is increased in such a case, most of the red light and blue light contained in it are absorbed by the chloroplasts that have reached the light saturation on the front side, and most of the energy is dissipated as heat. Become. On the other hand, green light, which is difficult to be absorbed by chloroplasts, should reach deep into the leaves and drive photosynthesis of chloroplasts that have not reached photosaturation. Based on this idea, we devised a method to measure the increase in photosynthetic rate at that time by adding weak monochromatic light to strong white light, and named it "differential quantum yield measurement method". The measurement principle is shown in Fig. 4. Under white light irradiation with a certain photon flux density, the photosynthetic rate is first measured. Next, leaving the white light as it is, adding weak monochromatic light and dividing by the photon flux density (ΔI) of the monochromatic light obtained by adding the increment of the photosynthetic rate (Δ A) at that time is the differential quantum yield. (Φ = ΔA / ΔI). The specific measurement method is shown in FIG.
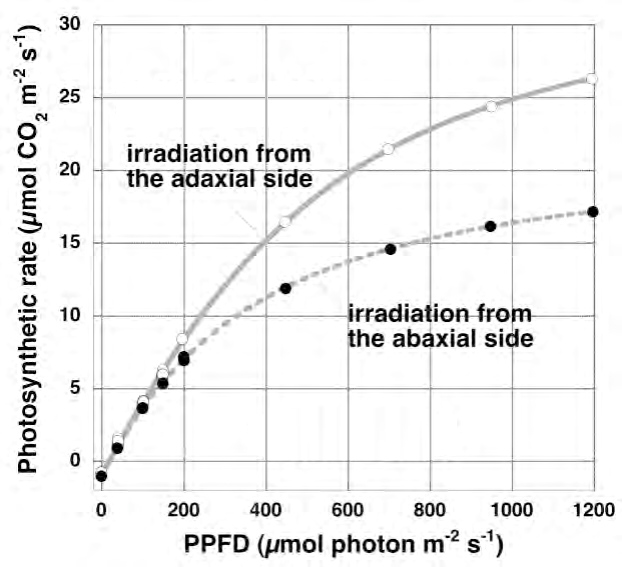
Fig. 5 Light-photosynthesis curve of the same sunflower leaf obtained by changing the irradiation direction is modified
Photo-photosynthetic curve measured at a leaf temperature of 25 ° C and an outside air CO2 concentration of 390 ppm. The curve differs greatly depending on the irradiation direction. When irradiated from the front side, the transition from the light rate-determining step to the light saturation step is sharper.
First, let's compare the light-photosynthesis curves of one sunflower leaf between the case of irradiating light from the front side and the case of irradiating light from the back side. As can be seen in Figure 5, the curves are very different. When irradiated from the back side, a lot of light is absorbed by the spongy tissue, and not much light reaches the positive chloroplasts existing on the front side. Therefore, in order to photosaturate all chloroplasts, extremely strong light is required. On the other hand, when light is irradiated from the front side, the differentiation of palisade tissue and spongy tissue and the gradient of positive chloroplast-negative chloroplast act in the direction that all chloroplasts reach photosynthesis at the same time. Therefore, the light-photosynthetic curve of the whole leaf becomes quite sharp. However, as already mentioned, these effects are not perfect.
The result of applying the differential quantum yield measurement method to this leaf is shown in FIG. The horizontal axis is the photon flux density of the irradiation light. Roughly speaking, the differential quantum yield corresponds to the slope of the photosynthetic curve, so it decreases with increasing photon flux density. When the light is irradiated from the front side, when the photon flux density is very small, the red light is more efficient than the green light, reflecting the difference in the absorption rate of the leaves. However, at 200 µmol m-2 s-1 and above, adding green light was more effective in increasing the photosynthetic rate than red light. When the light is irradiated from the back side, the green light is effective with the light much weaker than that. This is because the underlying tissue absorbs light more easily, and the chloroplasts reach light saturation with weak light.
The average quantum yield of monochromatic light in white light of a certain intensity is obtained by integrating the differential quantum yield from 0 to that light intensity. When actually calculated, when the light was irradiated from the front side, the average quantum yield of green light was larger than that of red light at 400 µmol m-2 s-1 or more. From these, it can be concluded that under strong light, green light drives photosynthesis more efficiently than red light and blue light. Of course, similar measurements need to be made for various leaves, but the average quantum yield of green light in nature may not be much different from that of red light.
In this way, the leaves use green light for photosynthesis quite well. Land plants continue to use chlorophyll, a pigment that does not easily absorb green light, probably because the efficiency of this pigment inherited from green algae was not bad. Of course, there is no reason why the hard-to-absorb wavelength range must be green.
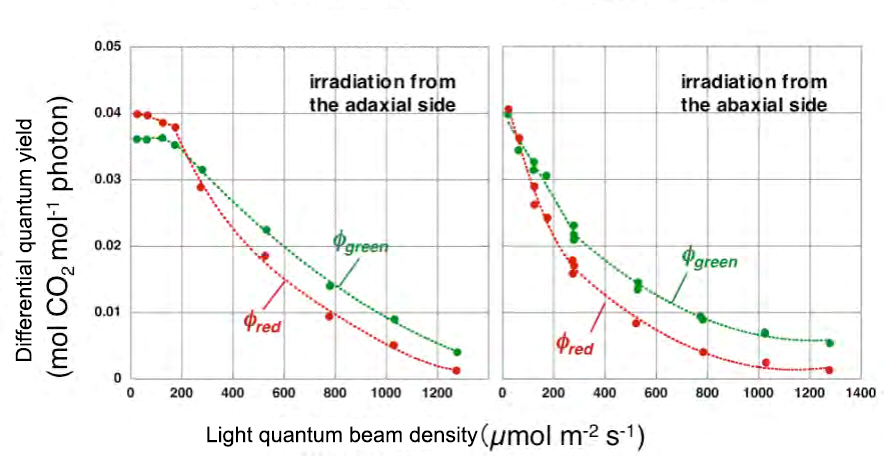
Fig. 6 Modified the light intensity dependence of the differential quantum yields of red light (550 nm) and green light (668 nm)
Measured under the same conditions as in Fig. 5. The photon flux densities of white light were 0, 40, 100, 150, 200, 450, 700, 950 and 1200 µmol m-2 s-1. The photon flux density of the added monochromatic light is 50 µmol m-2 s-1 when the white light is 150 µmol m-2 s-1 or less, and 200 µmol m-2 s-1 or more when the white light is 200 µmol m-2 s-1 or more. It was 150 µmol m-2 s-1. The left side is when irradiated from the front side, and the right side is when irradiated from the back side.
Mills that live at the bottom of the band-shaped distribution of green algae on the rocky shore appear black because they have a carotenoid called cihonaxanthine that can absorb green light and be used efficiently for photosynthesis. Therefore, it is possible to produce black chloroplasts even if it has chlorophyll as the main photosynthetic pigment. In dark places, it would be better to have black chloroplasts. The light is so strong on land that black leaves may not have been produced. However, it may be worth a careful analysis of pigments such as land plants that prefer extremely dark light environments.